- Diagnostics
- 1 min read
Booster shot debated in US amid Covid resurgence
The federal government's push to introduce widespread Covid-19 booster sshots faced a test as a Food and Drug Administration (FDA) advisory panel met on Friday to weigh evidence on the extra jabs, a topic that has divided health officials, Xinhua news agency reported.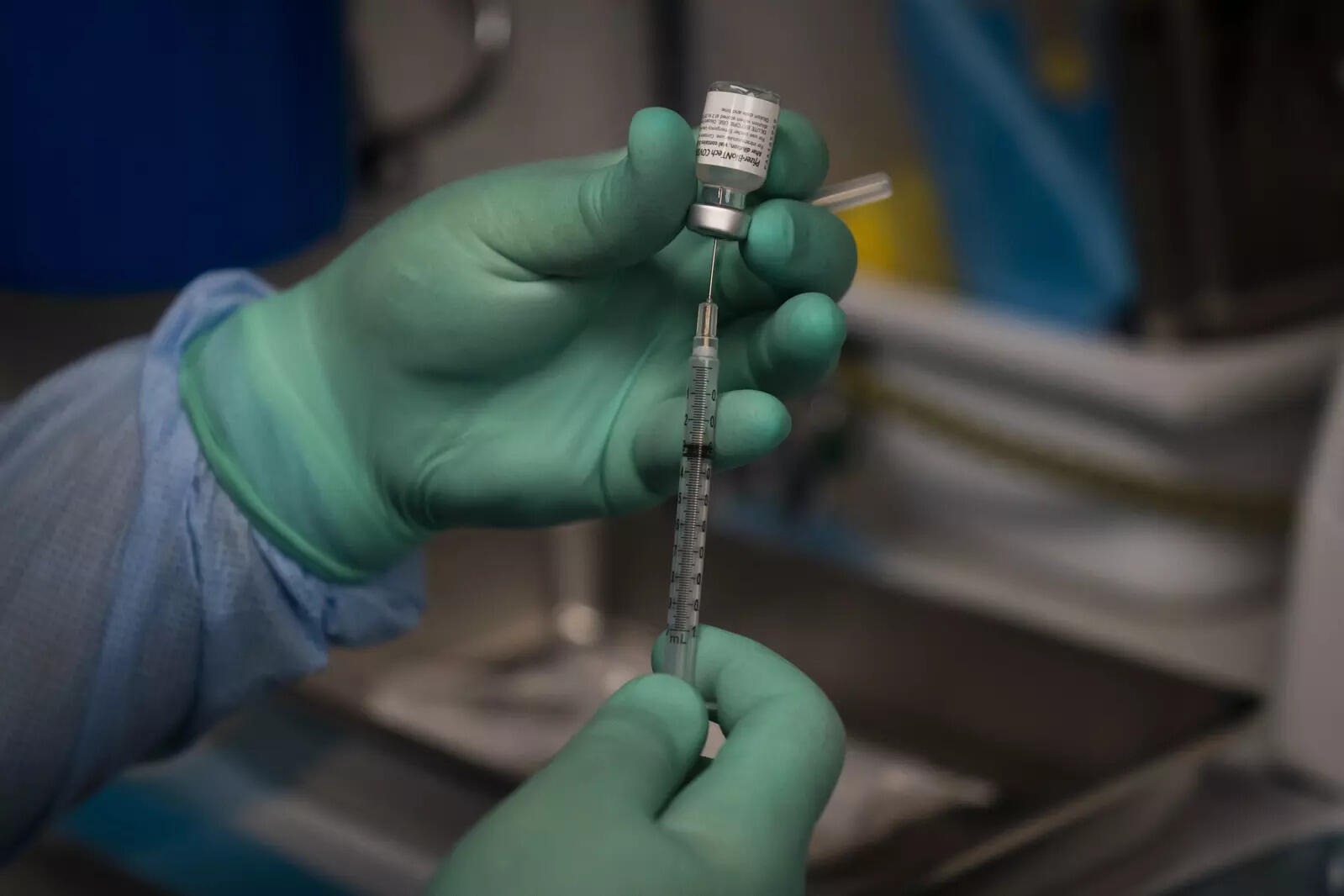
The federal government's push to introduce widespread Covid-19 booster sshots faced a test as a Food and Drug Administration (FDA) advisory panel met on Friday to weigh evidence on the extra jabs, a topic that has divided health officials, Xinhua news agency reported.
The outside panel of about 20 scientific advisers reviewed information about the Pfizer Inc. and BioNTech SE vaccine's declining protection against Covid-19 over time and on how people tolerated additional doses.
Pfizer has asked regulators to clear booster shots for people 16 years and older, and the FDA, which this week said all the shots cleared for use in the country remain effective without boosters.
While US health officials, some other countries and vaccine makers have said boosters were needed, many scientists, including some inside the FDA and the CDC, disagree.
The FDA panel then voted to recommend Covid-19 booster shots for Americans 65 and older and those at high risk of severe illness, but rejecting a broader use.
"Anything short of a full-throated endorsement could complicate the Biden administration's plan to begin distributing extra shots next week to bolster immunity among the vaccinated and counter highly transmissible variants of the virus such as Delta," The Wall Street Journal said in a report on Friday.



COMMENTS
All Comments
By commenting, you agree to the Prohibited Content Policy
PostBy commenting, you agree to the Prohibited Content Policy
PostFind this Comment Offensive?
Choose your reason below and click on the submit button. This will alert our moderators to take actions