- Policy
- 2 min read
Panel to fix compensation amount for those who received faulty hip implants reconstituted
The ASR hip implants were manufactured by M/s DePuy lnternational Limited, UK and imported by M/s DePuy Medical Private, Mumbai -- now Johnson and Johnson Private Limited -- according to the notice.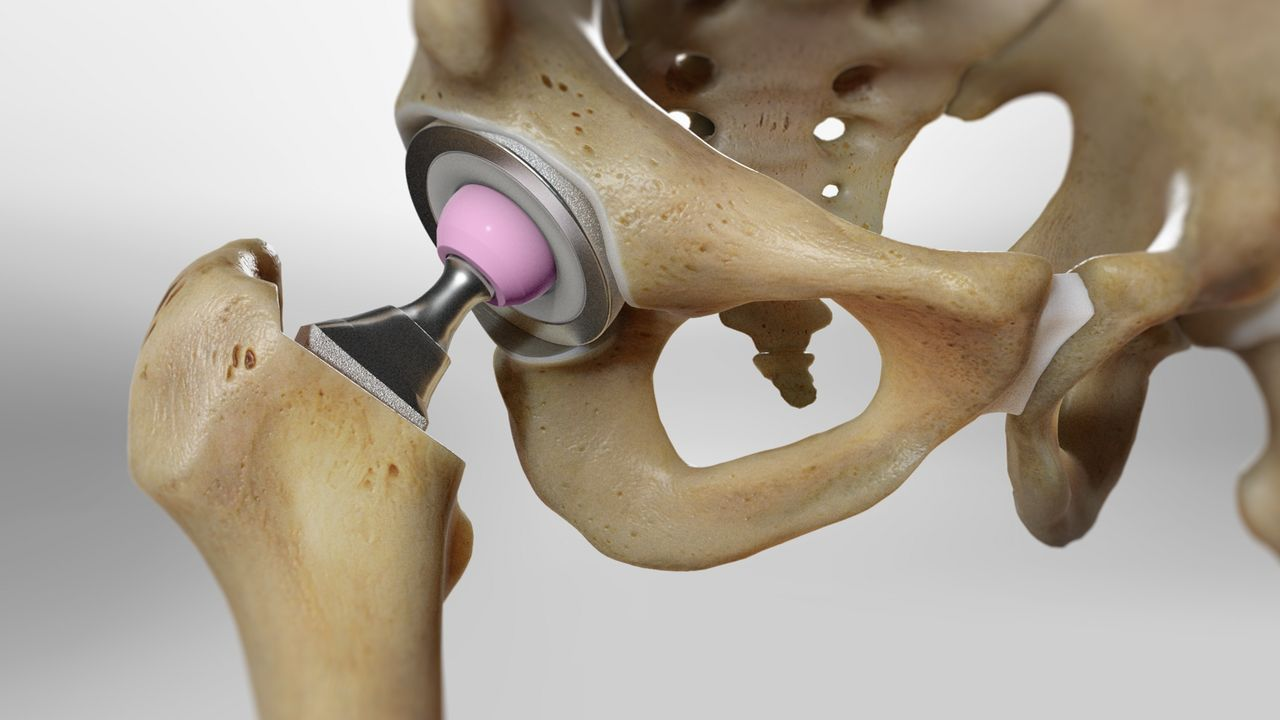
"The central expert committee is reconstituted under the chairmanship of Dr Ajay Kumar Shukla, Professor of Orthopaedics, RML Hospital (New Delhi), to determine the quantum of compensation as admissible and medical management for the affected patients who were implanted with faulty ASR hip implants," the notice read.
The ASR hip implants were manufactured by M/s DePuy lnternational Limited, UK and imported by M/s DePuy Medical Private, Mumbai -- now Johnson and Johnson Private Limited -- according to the notice.
In 2010, the company issued a global recall on the product following complaints related to hip implants from other parts of the world.
The affected patients who were implanted with faulty ASR hip implants and are suffering from disability and other losses may approach either the Central Expert Committee or the state-level committee in accordance with their convenience.
"In case the affected patients intend to approach the Central Expert Committee, they may write an e-mail or send a hard copy by post/by hand to Legal Cell, CDSCO (HQ), FDA Bhawan, Kotla Road, New Delhi-110002, Email ID: [email protected]," the notice said.
The ASR patients who have already submitted their claim applications before the state or Union Territory-level committee or the Central Expert Committee are not required to file any fresh claim application.
In case the affected patients intend to approach the state-level committee, they may write to the state or Union Territory drugs controller concerned, who will be the member-secretary of the state-level panel, the notice said.



COMMENTS
All Comments
By commenting, you agree to the Prohibited Content Policy
PostBy commenting, you agree to the Prohibited Content Policy
PostFind this Comment Offensive?
Choose your reason below and click on the submit button. This will alert our moderators to take actions