- Pharma
- 1 min read
Zydus Cadila gets DCGI nod to initiate Phase-3 clinical trials for Covid-19 vaccine
The phase II study of ZyCoV-D had been conducted in over 1,000 healthy adult volunteers as part of the adaptive Phase I/II dose-escalation, multi-centric, randomised, double-blind placebo-controlled study, the drug firm said.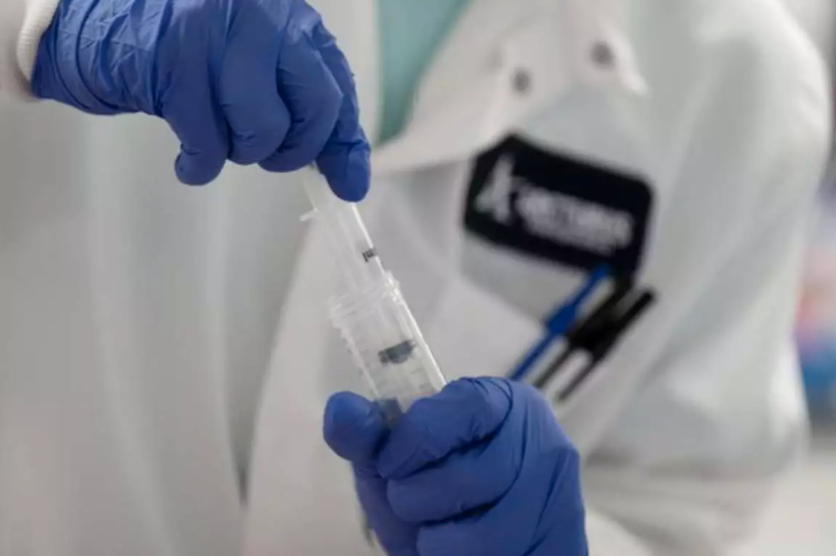
ZyCoV-D was found to be safe, well-tolerated and immunogenic in phase I and II clinical trials, it added.
The phase II study of ZyCoV-D had been conducted in over 1,000 healthy adult volunteers as part of the adaptive Phase I/II dose-escalation, multi-centric, randomised, double-blind placebo-controlled study, the drug firm said.
The trial has reviewed by an independent data safety monitoring board (DSMB) and reports were submitted to the Central Drugs Standard Control Organisation (CDSCO) regularly for the update on safety outcome.
"We are reaching a critical milestone in our vaccine development programme and towards our goal of helping people fight the pandemic with an indigenously discovered, safe and efficacious vaccine," Zydus Group Chairman Pankaj R Patel said.
The launch of the Phase 3 trial will determine the efficacy of the company's vaccine in preventing Covid-19, which continues to pose a major threat world over, he added.
The Drug Controller General of India (DCGI) on Sunday granted emergency approval to Serum Institute and Bharat Biotech for their respective vaccines.

Comments
All Comments
By commenting, you agree to the Prohibited Content Policy
PostBy commenting, you agree to the Prohibited Content Policy
PostFind this Comment Offensive?
Choose your reason below and click on the submit button. This will alert our moderators to take actions