- Pharma
- 1 min read
US FDA declines to approve Ascendis' hormone disorder therapy
Ascendis' US-listed shares rose 12 per cent in premarket trade. The FDA, early last month, had identified unspecified deficiencies in the company's application for approval of the therapy, TransCon PTH. TD Cowen analyst Yaron Werber had raised concerns about the lack of clarity around FDA's decision in a note on Friday.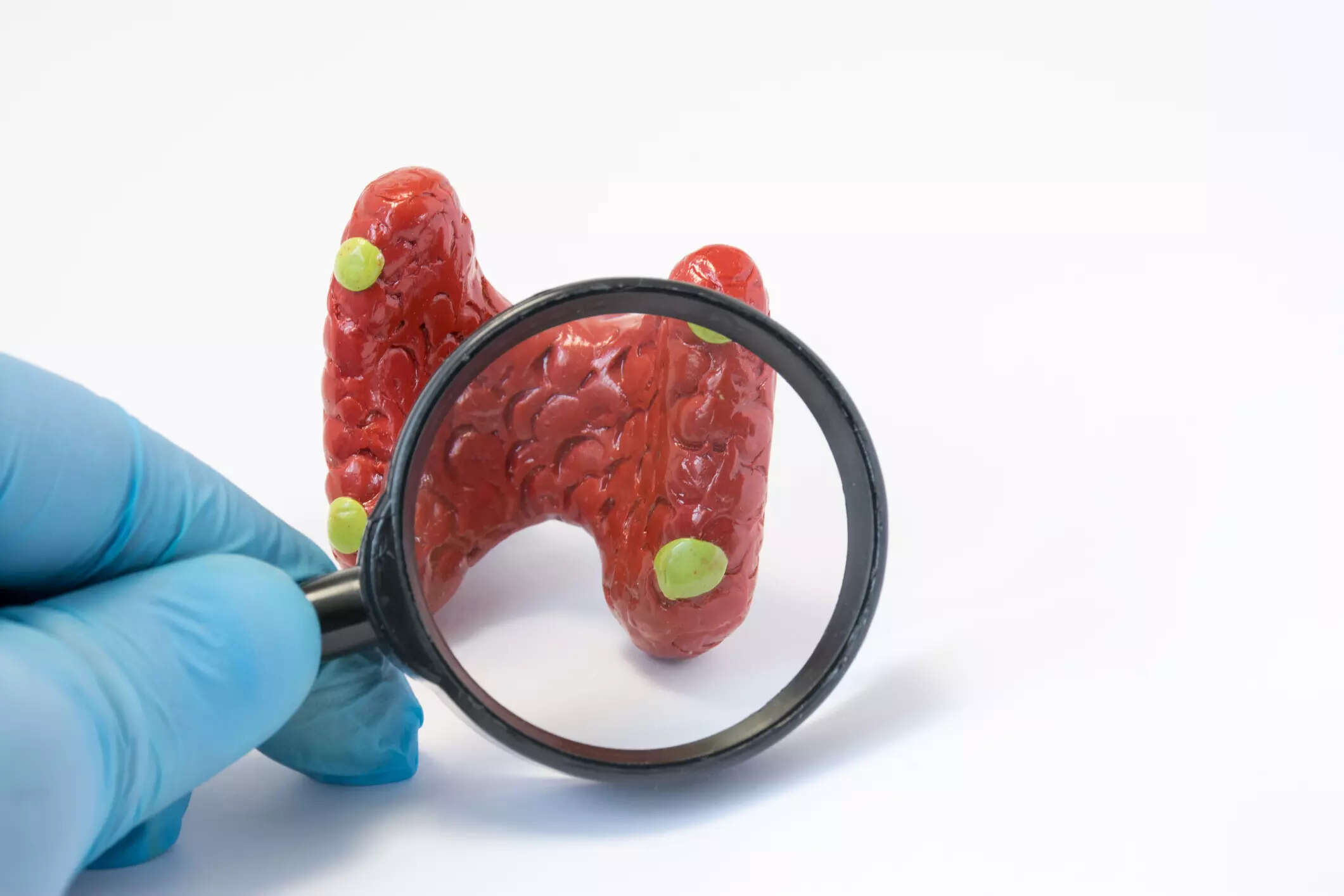
Ascendis' US-listed shares rose 12 per cent in premarket trade. The FDA, early last month, had identified unspecified deficiencies in the company's application for approval of the therapy, TransCon PTH.
TD Cowen analyst Yaron Werber had raised concerns about the lack of clarity around FDA's decision in a note on Friday.
He said a complete response letter declining approval was likely, adding the stock will slump if the FDA sought another pre-clinical or clinical study as this would delay approval by 1.5-2 years.

Comments
All Comments
By commenting, you agree to the Prohibited Content Policy
PostBy commenting, you agree to the Prohibited Content Policy
PostFind this Comment Offensive?
Choose your reason below and click on the submit button. This will alert our moderators to take actions