- Pharma
- 1 min read
Sanofi's, Regeneron's Dupixent wins new stage of EU regulatory approval
The European Commission is expected to announce a final decision on the Dupixent application in the coming months. Dupixent was approved in June 2022 by the U.S. Food and Drug Administration (FDA) regulator for children as young as six months old.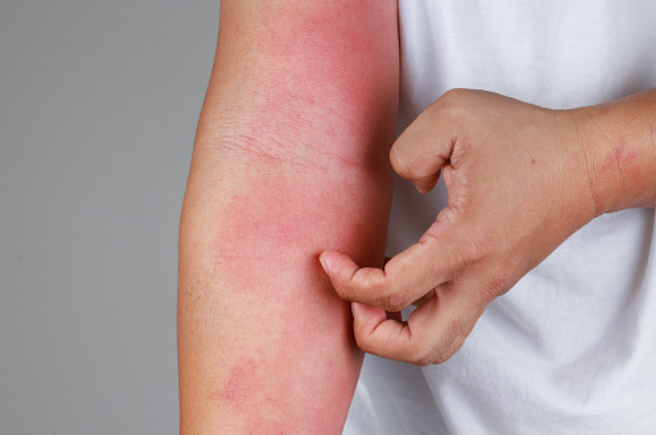
The European Commission is expected to announce a final decision on the Dupixent application in the coming months. Dupixent was approved in June 2022 by the U.S. Food and Drug Administration (FDA) regulator for children in this age group.
The use of Dupixent in infants and young children less than six years of age suffering from severe atopic dermatitis is at an investigational stage for now in the EU, pending final approval.
In October, Sanofi had forecast faster earnings growth due to strong demand for its bestselling drug Dupixent and for its flu vaccines.



COMMENTS
All Comments
By commenting, you agree to the Prohibited Content Policy
PostBy commenting, you agree to the Prohibited Content Policy
PostFind this Comment Offensive?
Choose your reason below and click on the submit button. This will alert our moderators to take actions