- Research & Development
- 1 min read
BioNTech is proceeding with COVID shot in line with WHO guidance
BioNTech is collaborating on the vaccine with Pfizer in markets outside of greater China. A WHO advisory group last week recommended that this year's COVID-19 booster shots be updated to target one of the currently dominant XBB variants.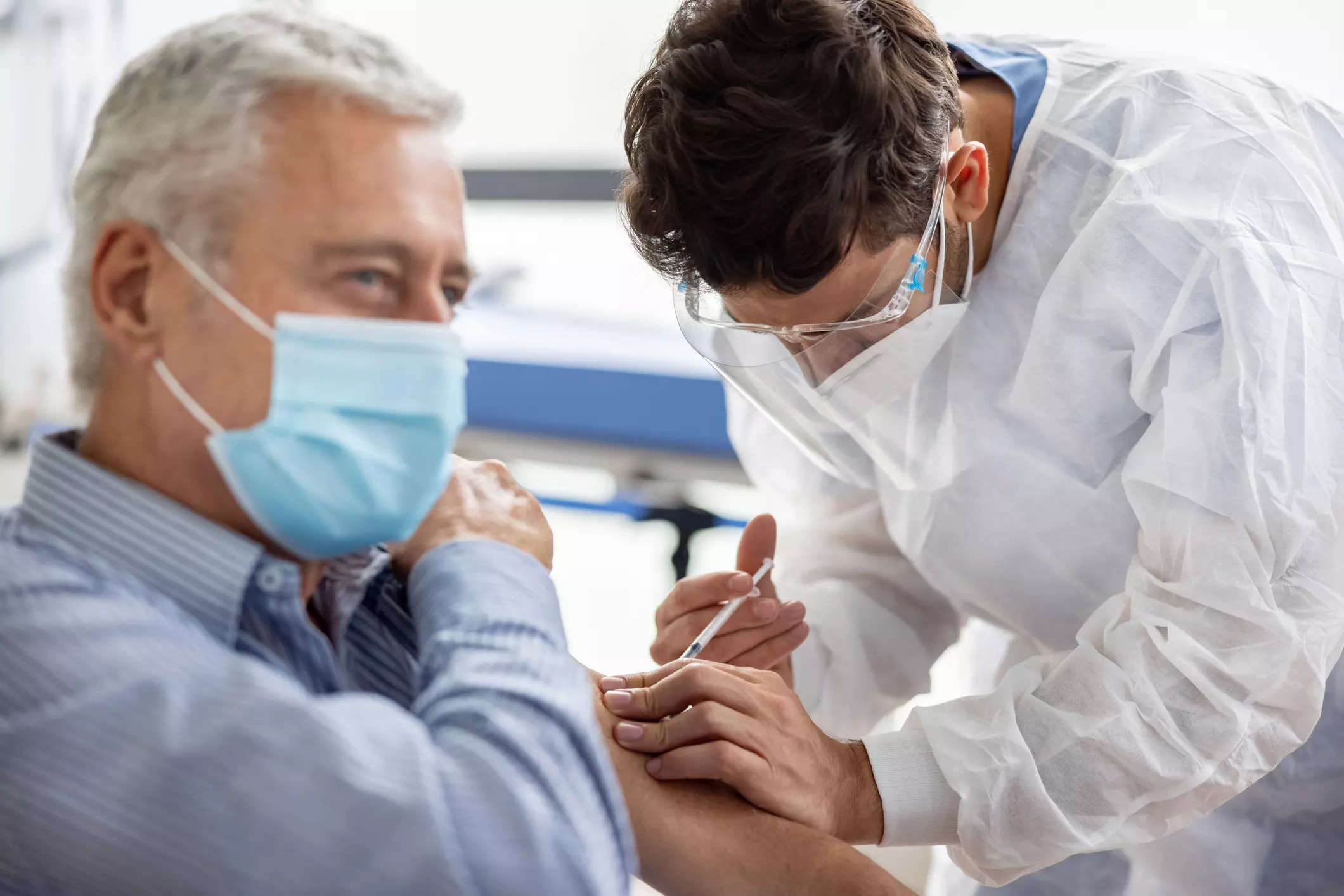
BioNTech was targeting regulatory approval by the end of the summer to allow for a seasonal vaccination campaign to start in early autumn, CEO and co-founder Ugur Sahin told shareholders at the biotech firm's annual general meeting on Thursday.
BioNTech is collaborating on the vaccine with Pfizer in markets outside of greater China. A WHO advisory group last week recommended that this year's COVID-19 booster shots be updated to target one of the currently dominant XBB variants.
BioNTech/Pfizer's new formulation would aim to produce antibody responses to the XBB.1.5 or XBB.1.16 variants, as preferred by the WHO, Sahin said, adding that the partners would introduce a ready-to-use single dose, a change from the multi-dose vials that were standard during the pandemic.
Moderna and Novavax have also been working on versions of their respective vaccines targeting XBB.1.5 and other currently circulating strains.



COMMENTS
All Comments
By commenting, you agree to the Prohibited Content Policy
PostBy commenting, you agree to the Prohibited Content Policy
PostFind this Comment Offensive?
Choose your reason below and click on the submit button. This will alert our moderators to take actions