- Pharma
- 3 min read
Lucknow: Central Drug Research Institute achieves breakthrough in trial of Umifenovir in Covid-19 treatment
The Central Drug Research Institute (CDRI) on Tuesday claimed that the clinical trials of antiviral drug, Umifenovir, in treatment of Covid-19 have been successful.LUCKNOW: The Central Dr 
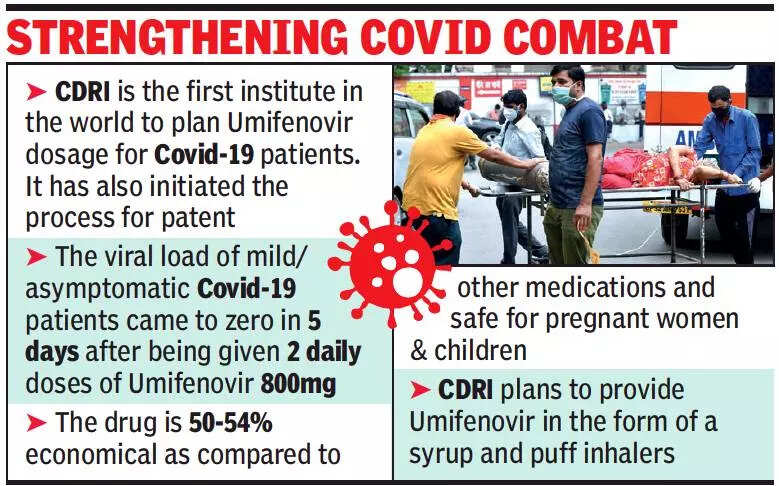
The trial of Umifenovir on 132 Covid-19 patients showed that, if proper dose is given twice daily for five days, the drug can effectively reduce viral load to zero in mild or moderate symptomatic and asymptomatic patients by checking multiplication of the virus.
Titled ‘Phase III, randomized, double-blind, placebo controlled trial of efficacy, safety and tolerability of antiviral drug Umifenovir vs standard care of therapy in non-severe Covid-19 patients’, the clinical trial was conducted at three institutions – KGMU, Ram Manohar Lohia Institute of Medical Sciences (RMLIMS) and Era’s Lucknow Medical College and Hospital (ELMCH).
“Since Umifenovir is a broad spectrum antiviral and is being used as a safe over-the-counter drug for influenza and pneumonia for over 20 years in Russia, China and other countries, the first two trials were not mandatory,” CDRI director Prof Tapas Kundu said.
“CDRI, therefore, directly went for phase-III trial, which was conducted on 132 patients who were either admitted to hospitals or were in home quarantine under the supervision of these hospitals,” CDRI director Prof Tapas Kundu said.
“Studies by CDRI in collaboration with CSIR-IMT, Chandigarh, also showed that Umifenovir exhibits good cell culture inhibition of SARS-Cov2, which suggests that the drug inhibits the entry of SARS-Cov2 virus into human cells,” Prof Kundu said.
He said the institute was getting the dosage plan patented as it had not been used earlier for Covid-19.
“The Drug Controller General of India (DCGI) has evaluated the clinical trials report and in view of the highly encouraging results, he has asked the team to continue the studies on more mild, asymptomatic patients for grant of emergency approval of the drug,” he added.
Chief scientist, CDRI, Prof R Ravishankar, who led the team of scientists, said: “Umifenovir will be economical for treating Covid-19 patients as it is around 50-54% cheaper as compared to current medication. Expert from the three hospitals which were part of the study said the drug is safe for pregnant women and children. We are looking into the possibility of Umifenovir syrup for children and also in powder form so that it can be used as puff inhalers.”
According to CDRI, the head of KGMU’s medicine department, Dr Virendra Atam, and medical superintendent, Dr Himanshu Reddy, who were principal investigators of the study at the university, mentioned in their report done for the study that faster recovery of coronavirus patients would reduce virus shedding and consequent spread of infection to others.
It also said that the principal of Era’s Medical College, Prof MMA Faridi, mentioned in his report that Umifenovir could be prescribed to pregnant women and children, if approved by the authorities.
Similarly, Prof Vikram Singh from RMLIMS suggested that as Umifenovir was safe, it had significant efficacy on mild and asymptomatic patients and could also be useful as a prophylactic for high-risk patients.
CDRI spokesperson Sanjeev Yadav said, “Umifenovir was selected from 16 drugs suggested by the CSIR after looking into the feasibility of synthesis using locally available chemicals at the peak of pandemic. The DCGI then gave permission for trials in June last year.”
A team of CDRI chemists, Ajay K Srivastava, Chandra Bhushan Tripathi, Nayan Ghosh and Nilanjana Majumdar, and their students, synthesized the drug and developed the process technology – chemical processing used to refine raw material into finished product – in record time.
The technology was then transferred to a Goa-based private pharmaceutical company within a month’s time to make the “active pharmaceutical ingredient” (API) and tablets for trials.
Finally, after securing ethical approvals and completing stability studies of the drug at CDRI, the team of researchers took consent of patients and roped them in for the study.



COMMENTS
All Comments
By commenting, you agree to the Prohibited Content Policy
PostBy commenting, you agree to the Prohibited Content Policy
PostFind this Comment Offensive?
Choose your reason below and click on the submit button. This will alert our moderators to take actions