- Pharma
- 2 min read
Jubilant Pharmova arm completes studies using novel oral formulation of Remdesivir
Jubilant has sought authorisation for additional studies for this novel oral formulation from the Drug Controller General of India (DCGI), it added.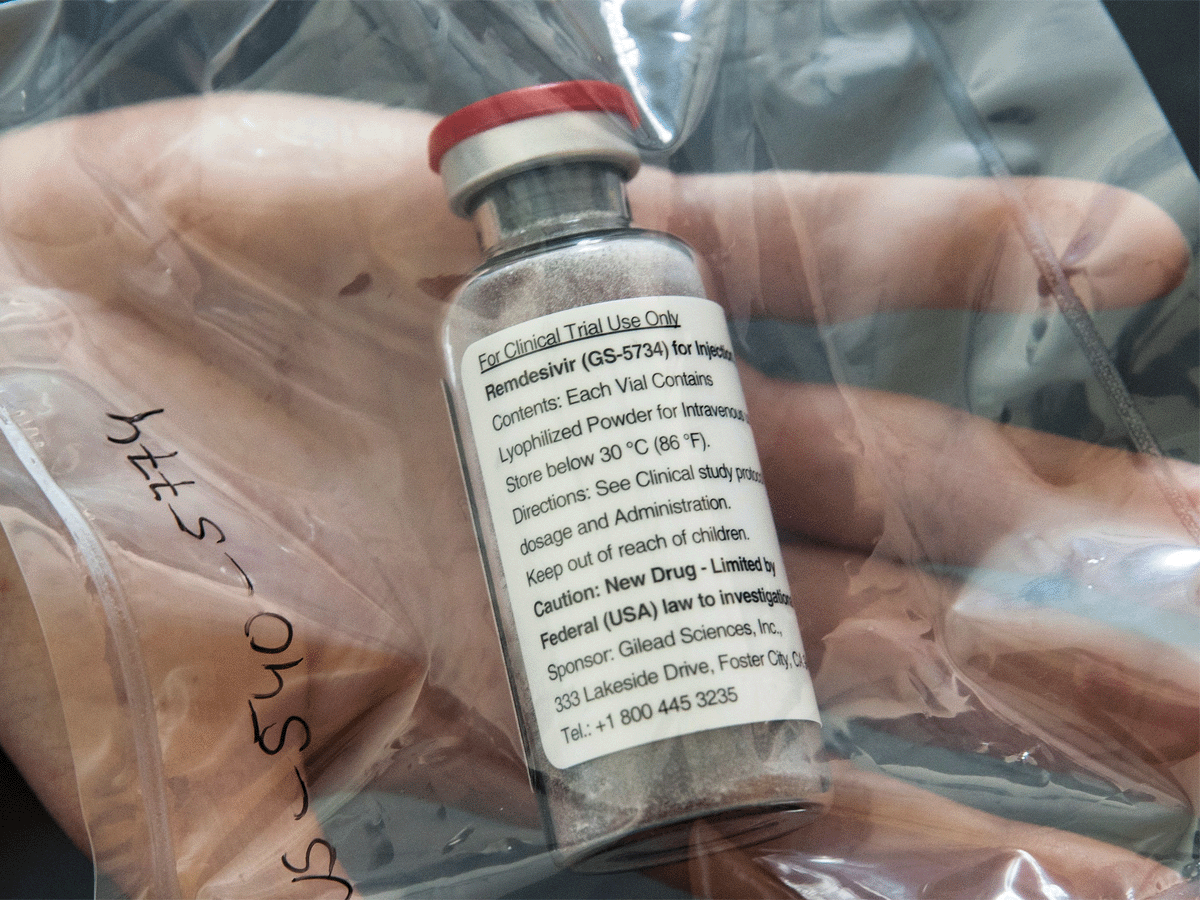
Jubilant has sought authorisation for additional studies for this novel oral formulation from the Drug Controller General of India (DCGI), it added.
"Jubilant is hoping to provide an affordable, more convenient, easy-to-administer and potentially effective treatment option for Covid-19 patients. The proposed oral treatment is expected to be for five days, a duration similar to the injectable dosage form," Jubilant Pharmova said.
Remdesivir is the first and the only anti-viral drug fully approved by the USFDA for the treatment of patients with Covid-19 requiring hospitalisation, it added.
"We are pleased to announce the ongoing development of a novel formulation of Remdesivir to address the pandemic at this critical juncture. Once approved, this will not only provide a more convenient and easy-to-administer formulation but also support an increasing demand of Covid-19 treatments," Jubilant Pharmova Chairman Shyam S Bhartia and Co-Chairman & MD Hari S Bhartia said.
In May 2020, Jubilant entered into a non-exclusive licensing agreement with Gilead Sciences, Inc, that granted it the right to register, manufacture and sell Gilead's Remdesivir in 127 countries including India, the filing said.
On July 20, 2020, Jubilant received approval from the DCGI to manufacture and market Remdesivir for 100 mg/vial for restricted emergency use in India for the treatment of severe Covid-19, it added.
Shares of Jubilant Pharmova were trading at Rs 775.30 per scrip on BSE, up 7.07 percent from its previous close.



COMMENTS
All Comments
By commenting, you agree to the Prohibited Content Policy
PostBy commenting, you agree to the Prohibited Content Policy
PostFind this Comment Offensive?
Choose your reason below and click on the submit button. This will alert our moderators to take actions