- Pharma
- 1 min read
Global Pharma under CDSCO watch after USFDA curbs eyedrops imports
Experts from DGCI are inspecting Global Pharma Healthcare's production facilities, and samples will be taken for further investigation, ET has been told by people aware of the developments.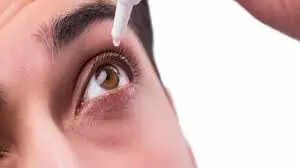
Experts from DGCI are inspecting Global Pharma Healthcare's production facilities, and samples will be taken for further investigation, ET has been told by people aware of the developments.
The products are not sold in India, the same people said.
Global Pharma Healthcare said it is notifying distributors Aru Pharma Inc and Delsam Pharma, and requesting wholesalers and retailers to recall the product. Customers who have the purchased the product should stop using it, the company said in a statement. This recall is being conducted with the knowledge of the US Food and Drug Administration, it further said.

Comments
All Comments
By commenting, you agree to the Prohibited Content Policy
PostBy commenting, you agree to the Prohibited Content Policy
PostFind this Comment Offensive?
Choose your reason below and click on the submit button. This will alert our moderators to take actions