- Pharma
- 2 min read
Cipla’s First Batch of Remdesivir dispatched from Sovereign Pharma
Remdesivir is known for successfully reducing the virus load in Coronavirus patients, and has been going through tests for specific treatment for COVID-19. It has been authorized for emergency use or severe cases in several countries including India.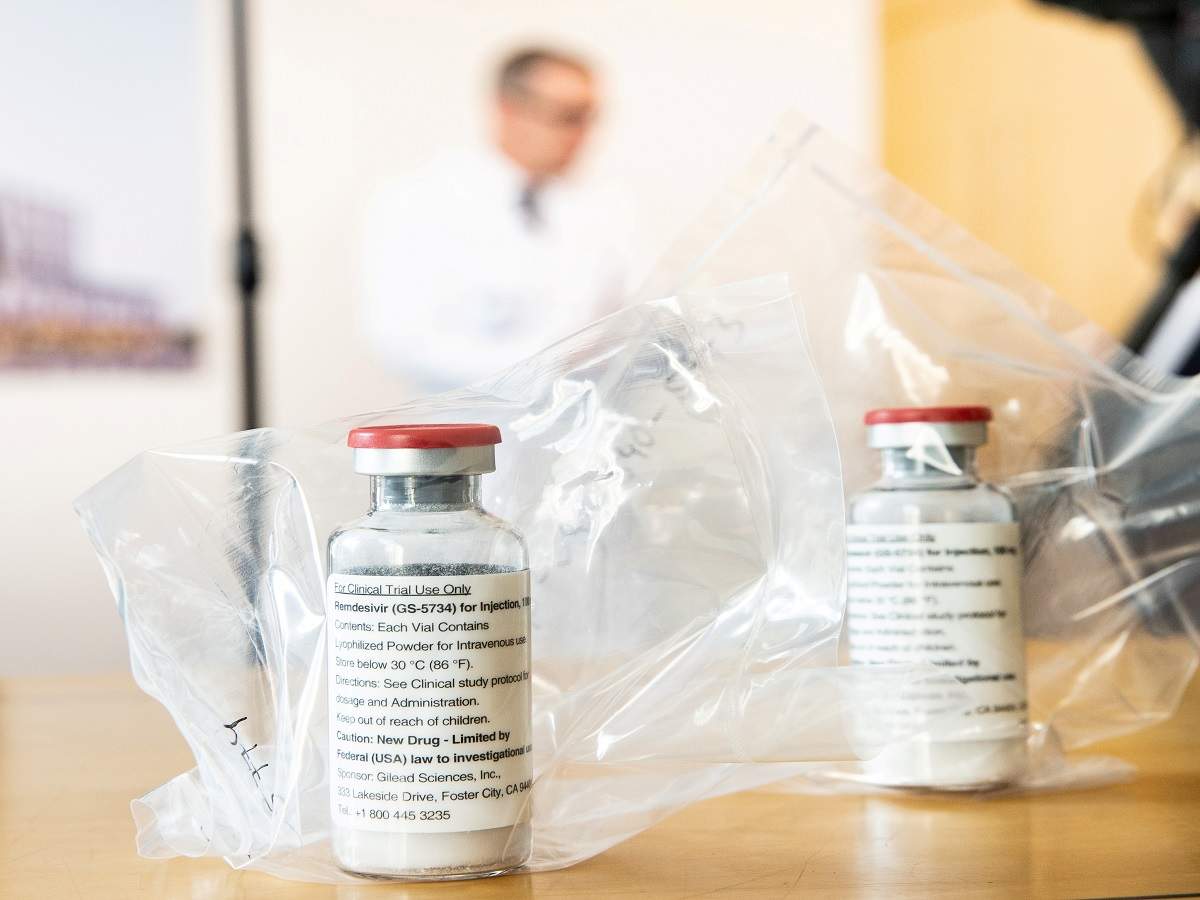
In absence of a definitive line of treatment, the Indian government under The Honourable Prime Minister Shri Narendra Modi has been instrumental in encouraging local drug manufacturers to identify potential treatments for the virus, as well as to ramp up production of the existing medications.
Within the union territory of Daman and Diu, one of India’s pharmaceutical hubs, Sovereign Pharma has prepared the first batch of Remdesivir within record time. Under the guidance of The Hon’ble Administrator Shri Praful Patel, the facility recently went through a meticulous inspection by the CDSCO and received necessary approvals from the authority within a week.
Remdesivir is known for successfully reducing the virus load in Coronavirus patients, and has been going through tests for specific treatment for COVID-19. It has been authorized for emergency use or severe cases in several countries including India.
Rishad Dadachanji, Director, Sovereign Pharma said, “We have been constantly manufacturing Remdesivir since last month, and at our current capacity, we can supply 50,000 to 95,000 pieces of the injectable per month. We know how critical the situation is, especially with the pandemic still spreading across the country. Hence, we are in constant touch with all our clients, and ready to cater to any of their requirements with utmost priority,”
With the PM Modi government’s push to become a global pharmaceutical hub, India has been at the forefront for producing and distributing effective medications to fight Coronavirus.
Cipla is amongst the six Indian generic pharma manufacturers that have signed non-exclusive voluntary licensing agreements with Gilead Sciences for its patented drug Veklury (Remdesivir). Cipla, in turn, has signed a contract manufacturing agreement with BDR Pharmaceuticals, who have further transferred the formulation technology for manufacturing and packaging of the generic drug to Sovereign.



COMMENTS
All Comments
By commenting, you agree to the Prohibited Content Policy
PostBy commenting, you agree to the Prohibited Content Policy
PostFind this Comment Offensive?
Choose your reason below and click on the submit button. This will alert our moderators to take actions