- Pharma
- 2 min read
Authorities cancel licences of several drug companies for lapses
Regulators had inspected dozens of drug manufacturing units in Himachal Pradesh, Uttarakhand, Haryana, Madhya Pradesh and Maharashtra, following reports of the death of 19 children in Uzbekistan allegedly caused by contaminated cough syrup made in India. According to the inspectors who carried out the inspections, certain products for which permissions were withdrawn were found to be not of standard quality.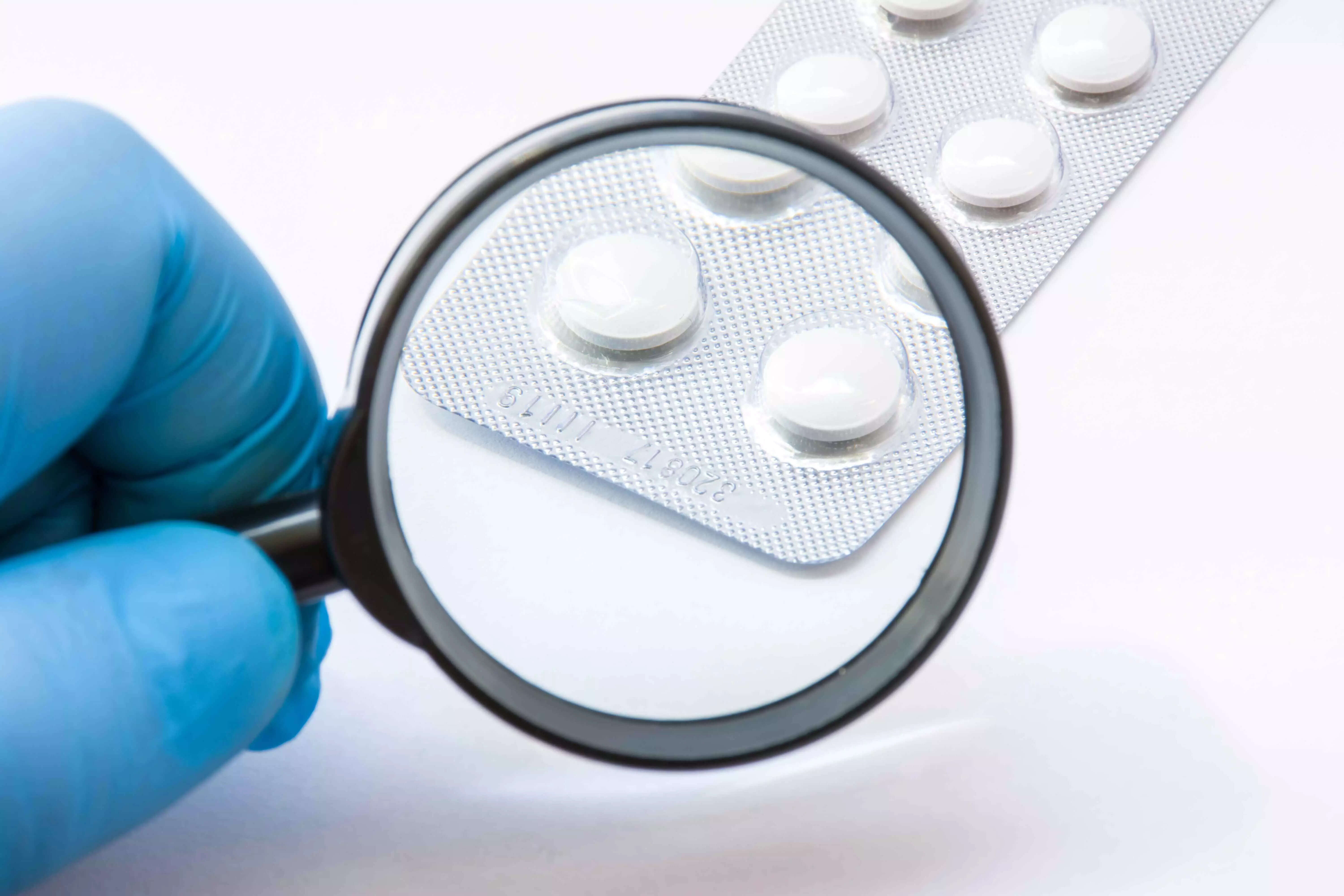
They took the action mostly during January and February. These manufacturers are accused of failing to comply with established good manufacturing practices.
ET has seen a copy of the report on the action taken by the licensing authorities. State licensing authorities took action against 34 drug manufacturing units: 17 in Himachal Pradesh, 13 in Uttarakhand, two in Madhya Pradesh and 1 each in Haryana and Maharashtra.
They cancelled the manufacturing licences of six units. These are of Himalaya Meditek, Mascot Health Series (for beta lactam section), SVP Life Sciences, Relief Biotech and Agron Remedies of Uttarakhand, and Sun Aj Pharma in Madhya Pradesh.
They also ordered some of the companies to halt manufacturing and suspended the product permissions of some others. These include Pure & Cure Healthcare, Skymap Pharmaceuticals, Anrose Pharma, GNB Medica Lab, Vintochem Pharmaceuticals, Apple Formulations, Relief Biotech, HAB Pharmaceuticals & Research, Rhydburg Pharmaceuticals, Bajaj Formulations and Trugen Pharmaceuticals.
According to the inspectors who carried out the inspections, certain products for which permissions were withdrawn were found to be not of standard quality.
Some units have been issued show-case notices and warnings. These include Shri Ramesh Industries, Athens Life Sciences, Laborate Pharmaceuticals India, Life Vision Healthcare, JM Laboratories, Park Pharmaceuticals, ANG Lifesciences India and Nestor Pharmaceuticals. The regulators revoked the stop-manufacturing notices against Shri Sai Balaji Pharmatech, Medipol Pharmaceutical, Alliaance Biotech, EG Pharmaceuticals, T&G Medicare and Zim Laboratories.
"We would like to inform that the company has neither received any show-cause notice nor any notice to stop manufacturing from any Drug Authority including DCGI or local drug authority," ZIM Laboratories said in a statement to stock exchanges on April 21.
"ZIM Laboratories adheres to current Good Manufacturing Practices as applicable and is accredited by Regulatory Authorities for marketing and distribution of several products in India and abroad," the statement added.

Comments
All Comments
By commenting, you agree to the Prohibited Content Policy
PostBy commenting, you agree to the Prohibited Content Policy
PostFind this Comment Offensive?
Choose your reason below and click on the submit button. This will alert our moderators to take actions