- Medical Devices
- 1 min read
NovoCure’s wearable device receives FDA approval for metastatic cancer treatment
The device, which disrupts cancer cell growth with electric fields, has shown improved survival rates in clinical trials with fewer side effects than traditional treatments.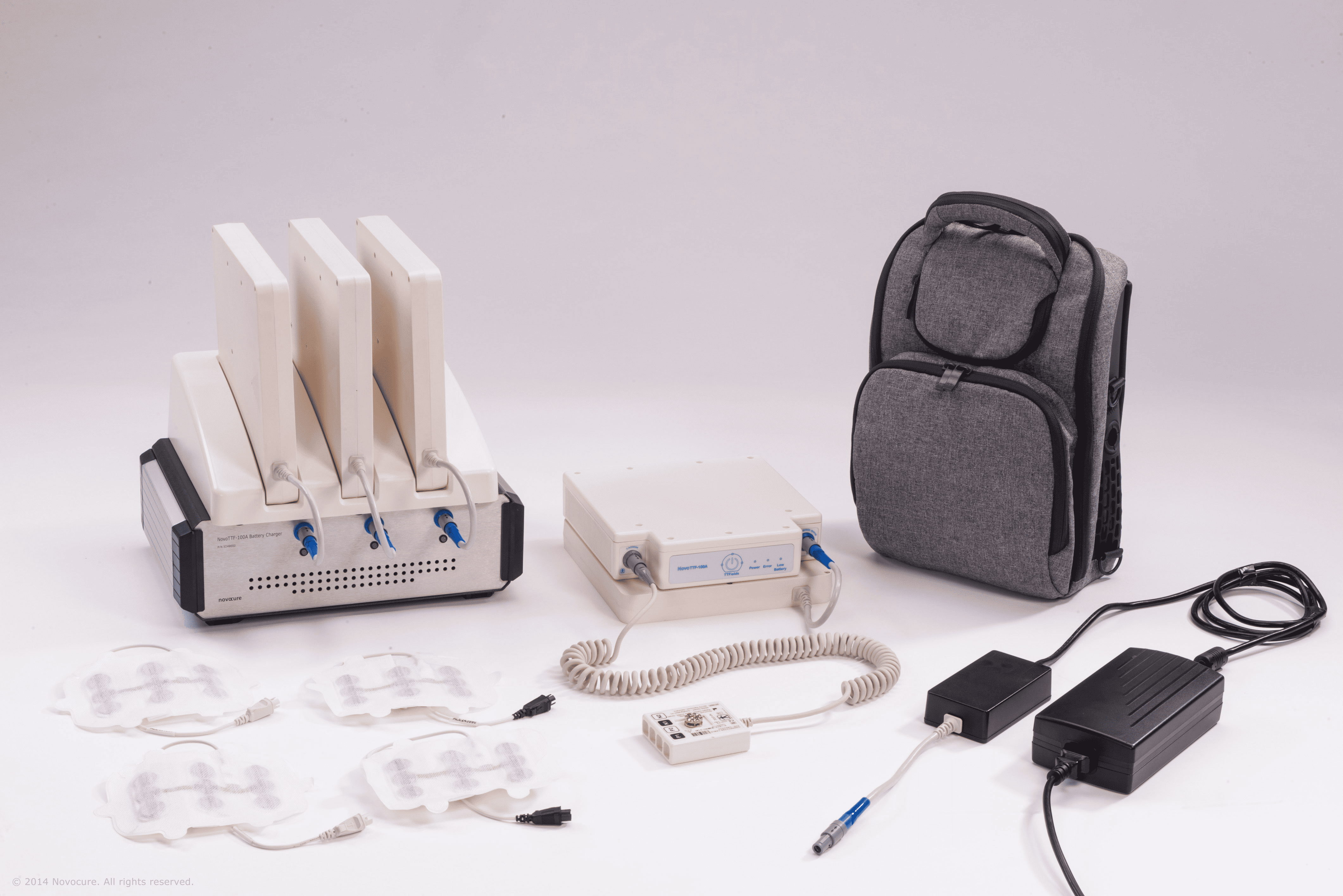
The global metastatic cancer treatment market is expected to grow from $63.03 billion in 2019 to $111.16 billion by 2027, driven by advancements in therapies like targeted treatment and immunotherapy. Increased research, investment, and initiatives in regions like India and Japan also contribute to this growth.
Clinical trials showed the device improved survival rates and had fewer side effects compared to chemotherapy and radiation. NovoCure is making the device available in the US and working with healthcare providers to incorporate it into cancer treatment plans.



COMMENTS
All Comments
By commenting, you agree to the Prohibited Content Policy
PostBy commenting, you agree to the Prohibited Content Policy
PostFind this Comment Offensive?
Choose your reason below and click on the submit button. This will alert our moderators to take actions