- Industry
- 1 min read
Remdesivir close to EU's initial authorisation as COVID-19 treatment
The European Medicines Agency (EMA) has already recommended the compassionate use of remdesivir, which allows a drug to be administered to patients even before it has been fully authorised.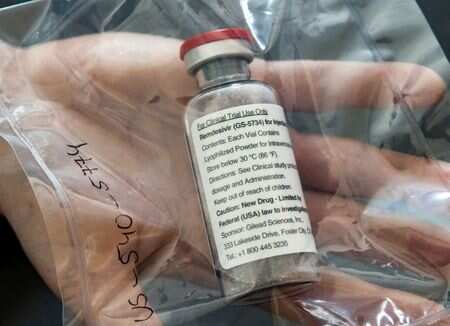
The European Medicines Agency (EMA) has already recommended the compassionate use of remdesivir, which allows a drug to be administered to patients even before it has been fully authorised.
"It might be that a conditional market authorisation can be issued in the coming days," Rasi told a hearing in the EU Parliament in Brussels.
Apart from remdesivir, Rasi said other possible treatments against COVID-19 that may be available fast are those based on monoclonal antibodies, which can "neutralize" the new coronavirus (SARS-CoV-2) that causes the illness COVID-19.

Comments
All Comments
By commenting, you agree to the Prohibited Content Policy
PostBy commenting, you agree to the Prohibited Content Policy
PostFind this Comment Offensive?
Choose your reason below and click on the submit button. This will alert our moderators to take actions