- Industry
- 2 min read
No more popping pills, now just inhale your antibiotics!
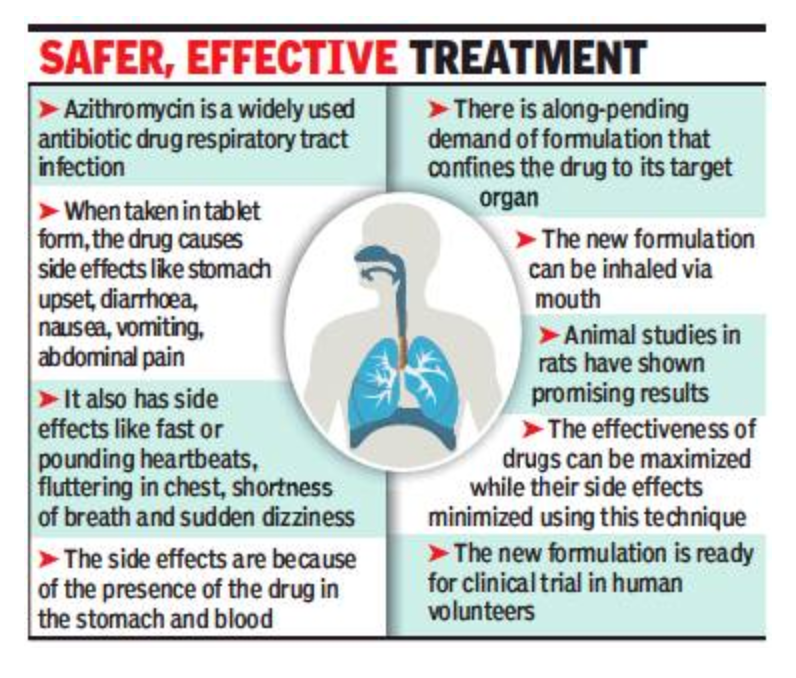
You may not have to pop up azithromycin pills in the future. Instead, you will be able to simply inhale this most common antibiotic drug widely prescribed to treat respiratory tract infections of the nose, throat and lungs.
Researchers of M S University’s Faculty of Pharmacy have received a patent for their invention of a liposomal dry powder inhaler (LDPI) of azithromycin. This invention will significantly reduce the side effects that the drug causes to people who consume it currently in pill form.
“We had taken up the project as there is a need for a better formulation for the treatment of lung infections. Lung infections are the most prevalent and leading cause of death from infectious diseases in the world,” said Dr Hetal Thakkar, who along with her students — Sunil Thakor and Dr Praveen Srivastava — carried out the research project.
“Azithromycin is a widely prescribed drug for the treatment of respiratory tract infections. But as the drug enters the stomach and blood, it causes a number of side effects. There has always been a demand for a formulation that can help the drug reach its target organ — the organ where the bacteria that is causing respiratory infection resides — without causing side effects,” said Thakkar.
The team developed the new formulation by encapsulating azithromycin within liposomes (used as a carrier), converting it into a dry powder by freeze-drying and filling it in capsules. Patients can easily take drug doses through dry powder inhalers.
The liposomal dry powder was evaluated using various in-vitro and animal studies. The in-vivo studies done on rats showed that the liposomes are retained in the lungs for a prolonged period of up to 12 hours with lesser presence in blood.
“These liposomes slowly released the loaded azithromycin into the lungs and thus avoided the exposure of the drug to stomach or blood,” she said.
“This helps in building local drug concentrations required for maximizing effectiveness and minimizing side effects,” said Thakkar, adding that the formulation is ready for clinical investigation in human volunteers.



COMMENTS
All Comments
By commenting, you agree to the Prohibited Content Policy
PostBy commenting, you agree to the Prohibited Content Policy
PostFind this Comment Offensive?
Choose your reason below and click on the submit button. This will alert our moderators to take actions