- Industry
- 1 min read
India's first intranasal vaccine for COVID-19 gets emergency use authorisation from DCGI
Bharat Biotech intranasal COVID-19 vaccine gets emergency use authorisation from DCGI . Union Health Minister Dr Mansukh Mandaviya call it to be a ‘Big Boost to India's Fight Against COVID-19’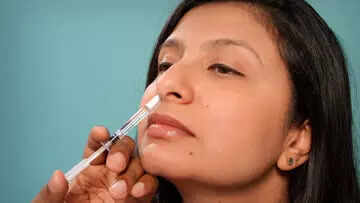
Bharat Biotech's ChAd36-SARS-CoV-S COVID-19 (Chimpanzee Adenovirus Vectored) recombinant nasal vaccine approved by CDSCO for primary immunization against COVID-19 in 18+ age group for restricted use in emergency situation.
Informing that this will be India's first nasal vaccine for COVID-19, Union Health Minister, Dr Mansukh Mandaviya termed it as a ‘Big Boost to India's Fight Against COVID-19’.
He further informed that the step will further strengthen India's collective fight against the pandemic.
"India has harnessed its science, R&D, and human resources in the fight against COVID-19 under PM @NarendraModi Ji's leadership. With the science-driven approach & Sabka Prayas, we will defeat COVID-19," he added.


Comments
All Comments
By commenting, you agree to the Prohibited Content Policy
PostBy commenting, you agree to the Prohibited Content Policy
PostFind this Comment Offensive?
Choose your reason below and click on the submit button. This will alert our moderators to take actions