- Diagnostics
- 3 min read
Maharashtra Receives Its State-Of-The-Art US-FDA Approved HPV Test For Primary Cervical Cancer Screening
Cervical cancer is the 2nd leading cause of cancer deaths amongst women in India and Maharashtra.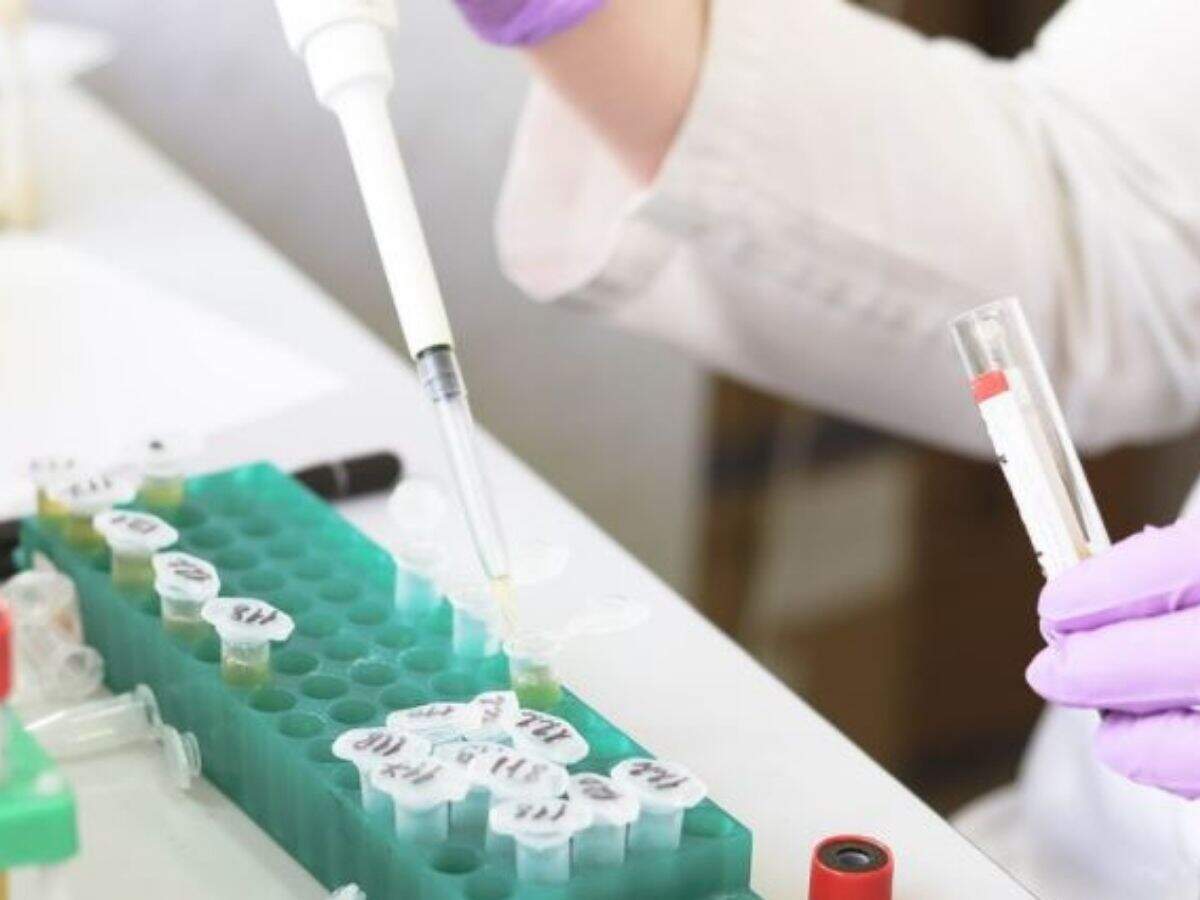
According various studies published, cervical cancer is the second leading cause of cancer death for women in the state of Maharashtra, reports ~10 deaths due to cervical cancer per 1000001. In 2019 ~5,700 deaths were reported due to cervical cancer.
In India, cervical cancer is the second most common cancer in women (aged 15–44 years) after breast cancer accounting for almost 14% of all women cancer. It is also one of the very few preventable cancers.
Due to insufficient accessible screening facilities, cervical cancer is detected late leading to high mortality rates. It is observed that screening programs conducted every 5 years in several countries have been able to reduce the incidence and mortality related to cervical cancer by 60%.
Mr. Manish Pungliya, Founder Director, AyuGen Biosciences, said, “Cervical Cancer is the only cancer whose cause is known & it can be prevented. At AyuGen our mission is to provide women opportunity to prevent & fight this cancer early - by providing them with high-quality & affordable HPV testing. Cervical cancer eradication is major challenge for our nation and the world in today’s time. Our organization has been working in this area of cervical cancer prevention for last 8 years and we are proud to launch state’s first US-FDA approved and clinically validated HPV DNA test. “Cervival” is the one-stop solution for Woman’s Health!”
Speaking on the occasion, Mr. Narendra Varde, Managing Director, Roche Diagnostics India and Neighboring markets, said, “Cervical cancer can be prevented. The enormous burden of mortality caused by cervical cancer brings to light the impending need to focus our attention on a program dedicated to it. This needs support of screening solution that provides the highest performance standards that Roche delivers. And the screening with HPV-DNA testing can more accurately identify women at risk for cervical cancer, helping in early detection and effective management of precancerous cases. We are happy to increase our footprint in India and enable access to a clinically validated screening test.”
“Cervical Cancer is preventable but yet not prevented in India. We must urge every gynecologist to do at least one screening per day preferably with HPV-DNA test for early diagnosis of cervical cancer. Cervical cancer is treatable and curable if detected in early stages,” said, Dr. Harshad Parasnis, Consultant Gyn Oncologist, President Pune ObGyn Society 2020 - 2021
WHO, aims to eradicate cervical cancer in 2030 and screen 70% of the eligible women to achieve this target. Recently, WHO launched a Global Strategy to Accelerate the Elimination of Cervical Cancer outlining three key steps: vaccination, screening and treatment. Successful implementation of all three could reduce more than 40 per cent of new cases of the disease and 5 million related deaths by 2050. The step to screening could be accomplished only by setting up of a scalable molecular HPV DNA test which is completely automated that will help in screening a huge population of eligible women.
The programme is aimed at providing access to primary HPV DNA test with genotyping from a dedicated center for testing at AyuGen Biosciences for 50000 women within next 5 years.
The HPV DNA test is the first US-FDA approved test for cervical cancer screening, being clinically validated it is the most commonly used test in national screening programs of several countries like Netherlands, Australia, Singapore, Malaysia to name a few. WHO aims to eradicate cervical cancer in 2030 and screen 70% of the eligible women to achieve this target. A scalable molecular HPV DNA test which is completely automated will be suitable to achieve screening a huge population of eligible women .


COMMENTS
All Comments
By commenting, you agree to the Prohibited Content Policy
PostBy commenting, you agree to the Prohibited Content Policy
PostFind this Comment Offensive?
Choose your reason below and click on the submit button. This will alert our moderators to take actions